HOME PRODUCTS LAB DIVISION
FOB5 Stand-alone Lab Sterilizer presents a great flexibility of use, ranging from quality assurance laboratories in pharmaceutical or food industries, to BSL3 – 4 laboratories in research centres and/or hospitals.
The possible application of FOB5 series of autoclaves can be:
- Microbiology and analytical labs.
- Research institutes and examination agencies.
- Bio-technologies and life sciences.
- Animal facility and clinical diagnostic labs.
- Pharmaceutical, food, chemistry industries, Q.C. labs.
- Agricultural, environmental and veterinary labs.
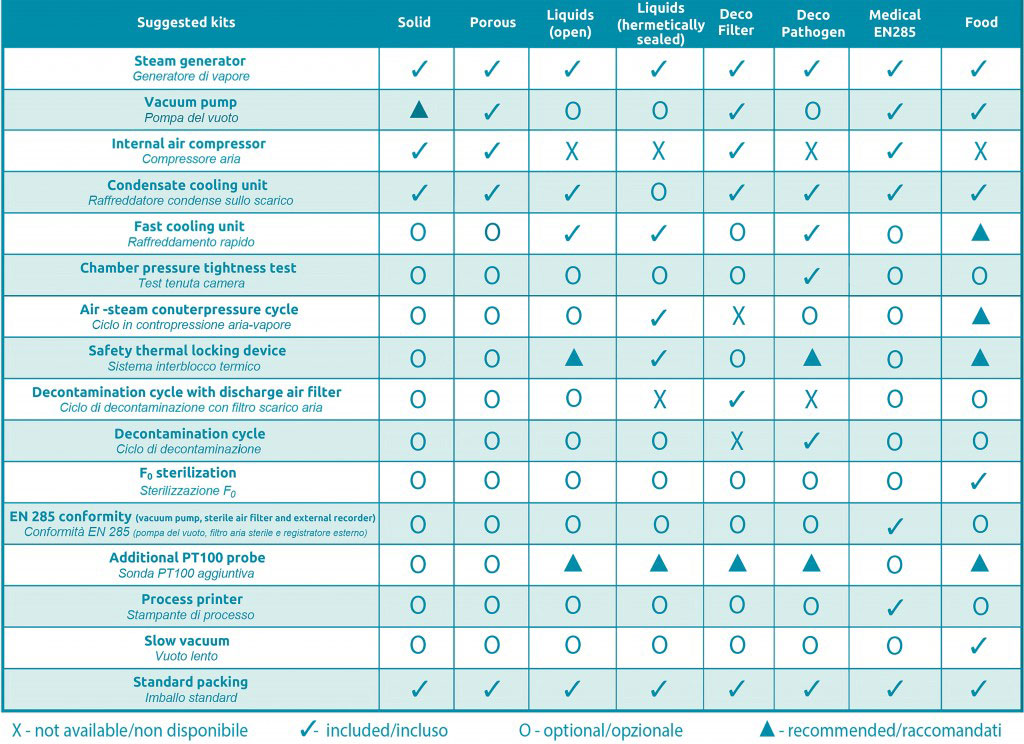
As all the autoclaves of Fedegari Lab Division, FOB5 has a modular design which allows a high flexibility of use for different loads and treatments. Please, click on the link “recommended kits” on the right to check out all the possible configurations referred to FOB5 series.
DESIGN AND TECHNICAL FEATURES
FOB5 autoclave series has a wide range of chamber volumes and come with both single and double vertical sliding door:
- FOB5C-TS: 362 liters – single vertical sliding door.
- FOB5-TS: 455 liters – single vertical sliding door.
- FOB5S-TS: 481 liters – double vertical sliding door.
- FOB5SL-TS: 590 liters – double vertical sliding door.
- FOB5L-TS: 615 liters – single vertical sliding door.
- FOB5SXL-TS: 7698 liters – double vertical sliding door.
- FOB5XL-TS: 729 liters – single vertical sliding door.
FOB5 series can be designed with a single or double stainless steel sealing flange (BIOSEAL), making it suitable for laboratory risk category L3 and L4 and operating theater. FOB5 Lab Sterilizers have two high-efficiency internal 316L stainless steel plates which can be used as heat-exchanger system for steam preheating, to cool the chamber temperature down by using cold water.
Moreover – as an option – these plates can be used to perform final drying.
FOB5 autoclaves are made of 316L stainless steel as well as pneumatic valves and hydraulic components.
Full and easy accessibility to technical area from the front and lateral side allows service and regular maintenance. External trolleys are fully compatible with FGW Fedegari Glassware Washer Series to assure integration between cleaning and sterilization processes in your laboratory.
FOB5 series is equipped with DCS20 process controller: 30 cycles easy to customize in a multi-user environment. Large touch-screen color display and interface for remote monitoring via Ethernet control. DCS20 process controller is fully validated and documented.
KEY BENEFITS
- Vertical position of filters avoiding frequent rupture.
- Thema4Lab process controller.
- GAMP5 compliance.
- High tech chamber and door construction.
- Suitable for BSL 3–4 laboratories.
- High process reliability.
- External trolleys fully compatible with FGW washers.
COMPLIANCE TO
- PED Directive 2014/68/UE – Pressure equipment
- Machine Directive Macchine 2006/42/EC
- EMC Directive 2014/35/UE
- LVD Directive 2006/95/UE
Want to know more about our certifications? Please visit the dedicated section or do not hesitate to contact us.
STANDARD
- EN ISO 12100 Safety of machinery – General principles for design – Risk assessment and risk reduction
- EN ISO 13857 Safety of machinery – Safety distances to prevent hazard zones being reached by upper and lower limbs
- EN ISO 13849-1 Safety of machinery – Safety-related parts of control systems – Part 1: General principles for design
- EN IEC 60204-1 Safety of machinery – Electrical equipment of machines – Part 1: General requirements
- EN IEC 61326-1 Electrical equipment for measurement, control and laboratory use – EMC requirements – Part 1: General requirements
- EN 61010-1 Safety requirements for electrical equipment for measurement, control, and laboratory use – Part 1: General requirements
- EN 61010-2-040 Safety requirements for electrical equipment for measurement, control, and laboratory use – Part 2-040 Particular requirements for sterilizers and washer-disinfectors used to treat medical materials
- EN 13445-3 Unfired pressure vessels – Part 3: Design
- EN 4126-1 Safety devices for protection against excessive pressure – Part 1: Safety valves
Standard compliance for non-EU countries
United States of America (USA)
- ASME (American Society of Mechanical Engineers) U & S Stamp
- UL 508 A Standard for Industrial Control Equipment
- NPFA 70 National Electrical Code (NEC
- NPFA 79 Electrical Standard for Industrial Machinery
People’s Republic of China
- SELO – Chinese Safety Regulation for Pressure Vessel
Russian Federation
- TR CU 032 On the safety of equipment operating under excessive pressure
- TR CU 010 Machinery Directive
Brazil
- NR 13 Boilers and Pressure Vessel
Malaysia
- >Department of Occupational Safety and Health (DOSH)
Singapore
- Ministry of Manpower (MOM)
DOWNLOAD
RELATED PRODUCTS
DCS20
The DCS20 process controller was specially designed for laboratory machines manufactured by Fedegari.
The result is a modular system that offers a wide range of functions and high reliability.
The system includes a supervisor module and other individual ones dedicated to specific functions such as: programs management, cycles, phases, configurations, and alarms for the module.
Options
- REMOTE GUI allows remote connection to a PC by Ethernet connection. This connection allows the operator to view the machine display on the desktop.
- REMOTE CONTROL allows remote connection to a PC by the Ethernet connection port. This connection allows the operator to fully interact with the machine. This excludes door closing and data backup operations.
Data management in compliance with the requirements of the 21 CFR PART 11 protocol.
Life cycle and Validation
DCS20 is designed, developed, tested, maintained, and validated according to a defined life cycle according to regulatory requirements stated in the current GMP.
Life cycle management complies with FDA 21 CFR Part 11, up to the current operational stage in defining product management practices in accordance with the principles of the GAMP5 guideline.
According to the system classification defined in GAMP 5 – Appendix M4 Categories of Software and Hardware, DCSPLUS20 is classified as:
- Software Category 4 – Configurable system
- Hardware Category 1 – Standard hardware components
Tests on software and hardware components are performed, during project and engineering activities, by applying functional risk analysis according to ALCOA+ principles, as defined in the Change Control procedure, dedicated to DCS20, part of the Fedegari Quality System.