HOME PRODUCTS INDUSTRIAL DIVISION
UNIQUE APPROACH TO LIQUIDS STERILIZATION
Sterilization of aqueous solutions in sealed containers (such as LVPs – Large Volume Parenterals, vials, PFS – Pre-filled syringes, blister packs, etc. ) share a common problem: the pressure inside the primary container is higher than the pressure of saturated steam outside at the same temperature. This can affect the product and/or the container itself.
Our unique approach to counterpressure sterilization:
- Flexibility: control of critical process parameters designed to assure the highest flexibility of the sterilization cycle and to prevent any undesired deformation/damage of the containers, while providing the desired thermal treatment.
- Total control: temperature and total pressure control (air + steam). A flow deflector and one or more magnetic drives guarantee a uniform mixture spread.
- Process optimization: load emerges dry and ready for the next process steps.
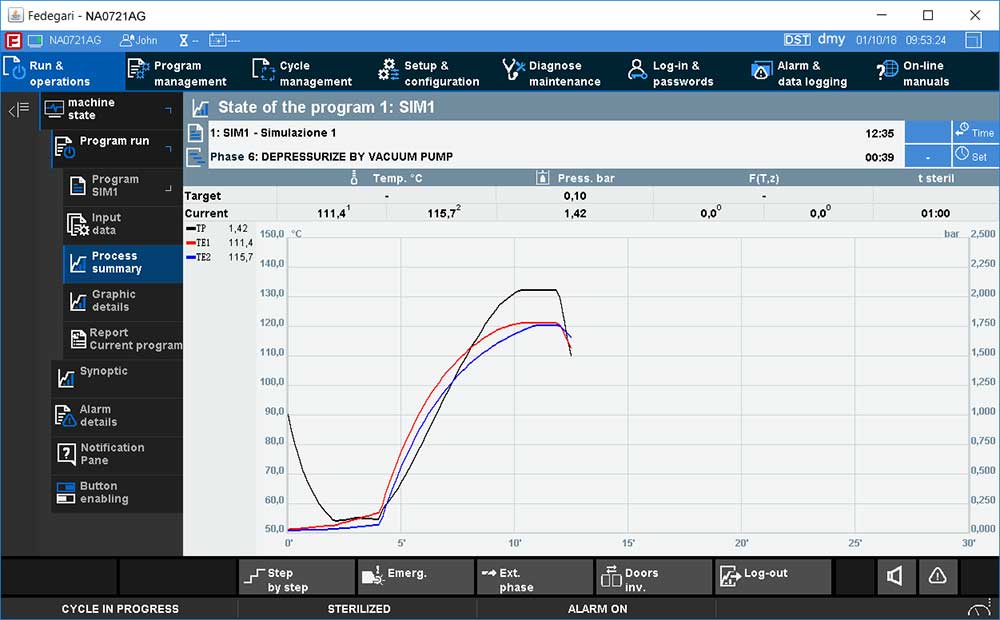
- Easy validation & integration: Thema4 process controller is pre-validated according to GAMP5 and in compliance with 21 CRF Part 11, thus resulting in shortest qualification time and minimizing project risks. Open system architecture that can be integrated with SCADA system and other Fedegari machines.
- Highest productivity: Fedegari R&D laboratory is available to develop the best treatment solution for your specific needs. We run tests with different loads and requirements.
DESIGN & TECHNICAL FEATURES
- Besides the standard versions from 830 to 14.800 liters, loading capacity is fully customizable according to customer needs.
- Chamber internal surface mechanically polished with a roughness Ra <0,4 μm.
- One or two doors in automatic sliding version (vertical or horizontal movement) or semi-automatic hinged version.
- 5 PT100 RTD probes for temperature control (2 fixed – in the filter and discharge – and 3 flexible for the load).
- Automatic sterilization of the air filter with fixed temperature probe.
- 2 validation ports.
- Fedegari exclusive patented door gaskets that assure 100% tightness. These gaskets are FDA compliant and optionally also USP Class VI compliant.
- Piping and valves in 316L – 1.4435 and 316L – 1.4404 stainless steel.
- Patented magnetic drives remove any tightness problem deriving from seal wear, and minimizes maintenance, without requiring any lubrication.
APPLICATION FIELDS
FOA steam air mixture sterilizer is suitable for:
- Sterilization of parenteral solutions, liquids in sealed containers not deformable (eg. glass vials) and liquids in heat-sensitive sealed containers (eg. plastic bags or bottles).
- Terminal sterilization of pre-filled syringes (PFSs).
SPECIAL APPLICATIONS
ROTATING LOAD
The load is held in rotation thanks to a special rotating drum inside the chamber. The rotation drum has a magnetic coupling and is adjustable in speed, direction of rotation and intermittently. This system finds application in the treatment of suspensions and emulsions and ensures a better performance of the tightness tests for ampoules under vacuum and a more homogeneous heat transmission in the load.
FULLY INTEGRATED TURN-KEY SOLUTIONS
Robotized handling system with FOA
STANDARDS COMPLIANCE
- European directives: 2014/30/EU – Electromagnetic compatibility (EMC), 2014/35/EU – Low tension equipment (LVD), 2006/95/EC – Safety of machinery (MD)
- European standards: EN ISO 12100, EN ISO 13857, EN ISO 13849-1, EN IEC 60204-1, EN 61326-1, EN 61010-1, EN 61010-2-040, EN 4126-1, EN 12953-9
- USA electrical standards: NFPA 70 – National electrical code, UL 61010-1 – Safety requirement for electrical equipmentfor measurement, control and laboratory use – Part 1: general requirements
- Pressure vessel and electrical steam generator compliant to: 2014/68/EU – Pressure equipment directive (PED)
- FDA: compliance for non metallic component in contact with process fluids
- EN 285
- GMP
- GAMP5
- 21 CFR Part 210, 211 e 11
- UL 508A
- NFPA-79
- ATEX 94/4/EC
- ASME BPE
- DIN 58951-2
Optional
- ASME: Stamp R, Stamp S, Stamp U
- Chinese standard: SELO
- Swiss standard: SR 930.114
- Russian standard: CU TR 032
- PD 5000 code specification for unfired, fusion welded pressure vessels
- USP: compliance for non metallic component in contact with process fluids
Want to know more about our certifications? Please visit the dedicated section or do not hesitate to contact us.