To enable cost-effective operations, pharma manufacturers must think holistically about their plants to develop integrated capabilities that connect systems and processes for remaining competitive. This way, in the last 10 years the integration trends of pharmaceutical plants ISA95 has been involving also process equipment such as sterilization, decontamination and washing machines.
Thema4 APPs
On this perspective, Fedegari Thema4 process controller provides customers with different applications presented as optional software APPs that range from workflow optimization to reliable integration with clients IT-network.
Built on the Thema4 platform, these ‘plug & play’ applications allow to improve the way Fedegari process equipment is used every day and provide ever-smarter functions for achieving business-critical performances in pharma manufacturing.
How to get started?
Choose the Thema4 APPs that best fit your needs
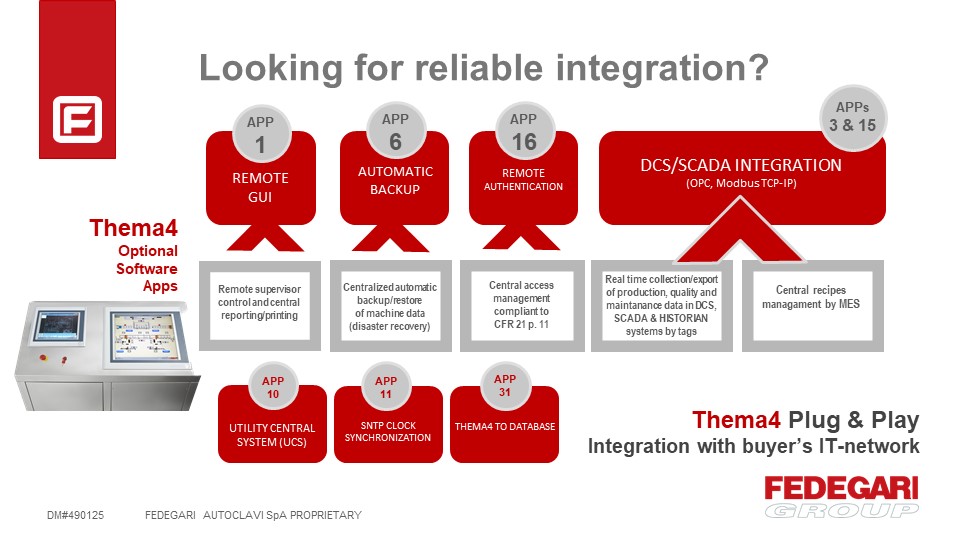
Thema4 RELIABLE INTEGRATION APPs
Recently, the planning capabilities of pharma industries have been significantly enhanced through new tools both within and on top of ERP systems. However, many companies still face difficulties on connecting systems, data compatibility, and other operational challenges. Thema4 APPs for integrated services aim to support customers in the reliable integration with their IT-network to achieve the highest performances and operate in the most cost-effective way.
APP #1 – Remote GUI (max 50)
Remote supervisory/control and central reporting and printing.
APP # 3 (OPC) and # 15 (MODBUS) – SCADA integration
Real time collection/export of production, quality and maintenance data in ER (Electronic Record): data base (tags), files (reports) and events collection for central audit trail and alarms management.
APP # 6 – Automatic Backup
Centralized automatic backup/restore of machine data (disaster recovery procedure).
APP # 10 – Utility Central System (UCS) Integration
APP # 11 – SNTP Clock Synchronization
APP # 16 – Remote Authentication
Central access management compliant to CFR21 part 11
(central password domain control manage by Active Directory).
APP # 31 – Thema4 to Database
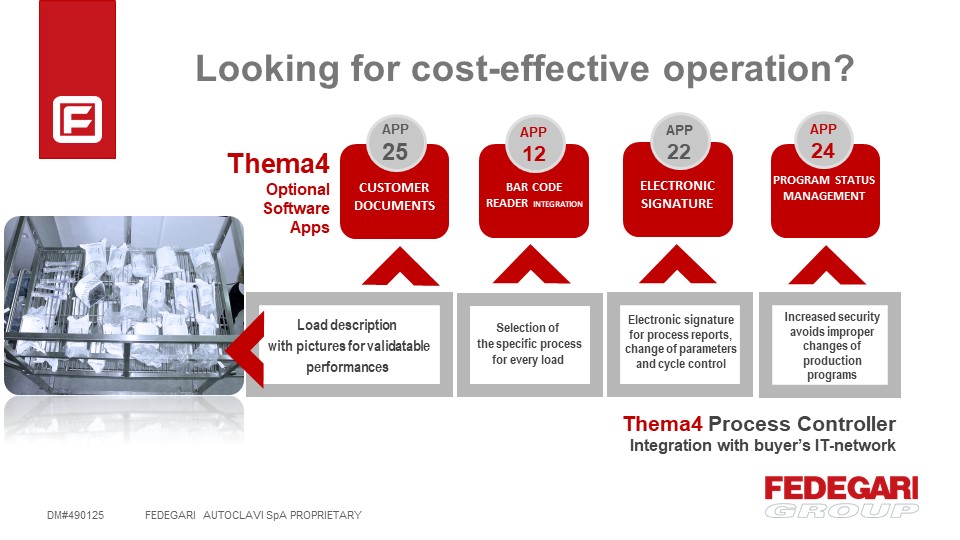
Thema4 COST-EFFECTIVE OPERATIONS APPs
A proper way to manage and select programs for defined loads. These applications concern important needs and challenges faced by operators of pharmaceutical plants.The ‘Cost-effective Operations’ APPs aim to minimize errors and to guarantee reliable operations while optimizing production processes.
APP # 12 – BAR CODE READER INTEGRATION
For programs selection and input of batch data; Correct selection of the program for a “defined load” in order to avoid load/machine damages.
APP # 22 – ELECTRONIC SIGNATURE
Electronic signature for process reports, change of parameters and cycle control.
APP # 24 – PROGRAM STATUS MANAGEMENT
Increased security in program management, in order to avoid improper changes of production programs or mistakes about “validation” and “production” programs.
APP # 25 – CUSTOMERS DOCUMENTS
Automatic features that help operators to assure a correct preparation of the load before running a cycle. Load description with pictures for validatable performances.
The correct execution of a treatment on a defined “load” also depends on the operator activities during load preparation, such as:
- How to position load/materials into the chamber
• Where to put temperature probes for process control and recording
• Where to put biological indicators, if any.
These activities must follow the validated approach defined for the load and documented in the customer procedure (SOP) adopted. The APP 25 “Customer Documents” allows the upload of customer documents in Thema4 system. Documents are displayed in the HMI work area “Online Manual”. A specific program parameter links the document to the treatment program, in order to display it after the program selection. Thema4 will permit the cycle to start only after an operator confirmation of the document viewed.
Thema4 “PARAMETRIC RELEASE” APPs
APP # 20 – GOLDEN CYCLE PACKAGE
Comparison of the temperature/pressure profiles of the executed process (using internal and/or external selected sensors) and the user-defined “ideal profiles” (as based on validated processes) and generation of an electronic report with comparison data in graphical and analytical format
APP # 21 – PARAMETRIC RELEASE DATA TABLE
Automatic analysis during cycle execution of “critical parameters” through user configurable phases and verification if the values do not exceed pre-set tolerances; generation of electronic reports with acceptance criteria (parametric release table available for SCADA and historian systems).
APP # 1 – INTEGRATION WITH YOKOGAWA SERIES
DX100P/DX200P AND DX1000P/DX2000P
APP # 14 – Thema4-RECORDER
External control system in compliance with EN285.
- Monitor, trend, diagnose, alarm, of both “local” and “recorder“ sensors.
- Data logging of recorder probes in Thema4 process report: a unique report for both systems, controller and recorder.
- Fully configuration of recorder sensors to Thema4 logic numbers (TPR, TER) and to the probes lists of the programs.
- Same calibration and SW-HW maintenance for both Thema4 controller and Thema4-RECORDER (by Remote GUI).
- Thema4 options available also for Thema4-RECORDER: Clock Synchronization SNTP and Remote Authentication.
APP # 19 – Integration with Eurotherm series 5000/6000
Thema4 Additional functions
APP # 26 – Animated P&ID
Interested?
Contact us for additional information on how to benefit from Thema4 APPs.
About Thema4
First delivered at the beginning of 2004, Thema4 is today the most reliable and fastest-developing process controller in the industry. Thema4 is defined as a completely configurable standard product (classified by GAMP5 as Category 4 – Configured Products) and is managing all industrial machines manufactured by Fedegari. This achievement is the logical consequence of almost three decades of painstaking work on process controllers combined with infrastructure investments, close collaboration with strategic partners and uncompromised values because there is no easy shortcut to durable success.
In order to guarantee the highest system reliability Fedegari has chosen Wind River VxWorks RT operating system. Over the years, collaborating with Wind River, has offered unequalled innovation opportunities impossible to be achieved alone.
In order to meet the most demanding pharmaceutical requirements, great care has been taken in designing Thema4 life cycle management: from the conception phaseto components selection and definition of system architecture and project management, through the project phase in the development of software according to the FDA CFR21, part 11, to the current operation phase and the definition of product management procedures (change control, configuration management, support, repair, maintenance, etc.) according to the GAMP guidelines.
Now, after continuous improvements of software and hardware components, functions and processes, Thema4 finds itself in the maturity of the operation phase and the reference guideline for its management is the GAMP5.
Several new software versions are released every year that include those developments requested or suggested by our end users. That’s why investing in a sterilizer with a Thema4 process controller allows the buyer to capitalize on decades of cumulative experience in managing pharmaceutical manufacturing processes developed for the most demanding companies worldwide.
An extensive team of experts assures that the controller is constantly innovated and manages all new developments in accordance with the end users needs and the adopted GMP standards.